

 Carbon
dioxide
Carbon
dioxide




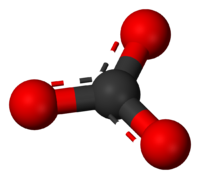

carbonate ion


hydrogen carbonate

Carbonato di sodio
Sodium carbonate
soda Solvay ?
"soda" or "natron"


carbonato di potassio


Carbonated water - Wikipedia, the free encyclopedia
| CO2 |
 |
 |
 |
 Carbon
dioxide Carbon
dioxide |
| CO |
 |
 |
||
| H2CO3 |
 |
 |
acido carbonico | |
|
CO3--
|
 |
 |
 |
ione carbonato carbonate ion |
 |
 |
ione bicarbonato hydrogen carbonate |
||
| Na2CO3 |
 |
Carbonato di sodio Sodium carbonate soda Solvay ? "soda" or "natron" |
 |
|
| K2CO3 |
 |
potash potassa carbonato di potassio |
 |
|
| NaHCO3 |
 |
bicarbonato di sodio, o idrogenocarbonato di sodio | ||
| KHCO3 | bicarbonato di potassio |


The Lewis structure of the carbonate ion has two (long) single bonds to negative oxygen atoms, and one short double bond to a neutral oxygen Simple, localised Lewis structure of the carbonate ion.
This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are equally long and that the three oxygen atoms are equivalent. As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance between three structures. This resonance can be summarized by a model with fractional bonds and delocalized charges:
bicarbonato di sodio, o idrogenocarbonato di sodio, o carbonato acido di sodio.
Sodium bicarbonate or sodium hydrogen carbonate